
United States Department of Agriculture
Agricultural Research Service

United States Department of Agriculture
Agricultural Research Service
| Rooster Semen Collection, Processing and Cryopreservation Protocol |

|

USDA ARS National Animal Germplasm Program
Collect semen samples from roosters by abdominal massage (Burrows and Quinn, 1937) and observed to ensure they are free of urates and feces.
Dilute samples immediately after collection 1:1 (vol:vol) with 5 °C glycerol-free Lake’s diluent (Lake and Stewart, 1978; recipe below).
Place diluted samples in a rack on ice and transport (<15 min) to a 5 °C room.
Slowly dilute samples 1:2 (vol:vol; sample to cryopreservation medium) with 5 °C Lake’s diluent containing glycerol (11 % final concentration, 5°C).
Load the samples into 0.5-mL straws.
Cryopreserve samples using the box freezing method: Samples are placed on a rack and frozen in liquid nitrogen vapor (6.4 cm above liquid nitrogen) for 10 min (cooling rate: 10 °C/min) and then plunged into the liquid nitrogen for storage (Phillips et al., 1996).
Thaw samples in a 5 °C water bath for 5 min (Phillips et al., 1996).
Glycerol is contraceptive to fertility with chickens and must be removed prior to insemination. Two methods of glycerol removal are described and both should be performed in a 5 °C room.
Step-wise dilution
Assumes an initial sample volume of 1.5 mL for this example:
Add glycerol-free Lake’s diluent equilibrated to 5 °C to the sample every minute in the following increments: 10 × 100 μL (10 dilutions of 100 μL per dilution), 10 × 200 μL, 10 × 500 μL, and 5
× 1000 μL (Tajima et al., 1989; Phillips et al., 1996; Purdy et al., 2009). Centrifuge the sample at 300 × g for 25 min.
Remove the supernatant, combine pellets if necessary and measure the sample volume and sperm concentration.
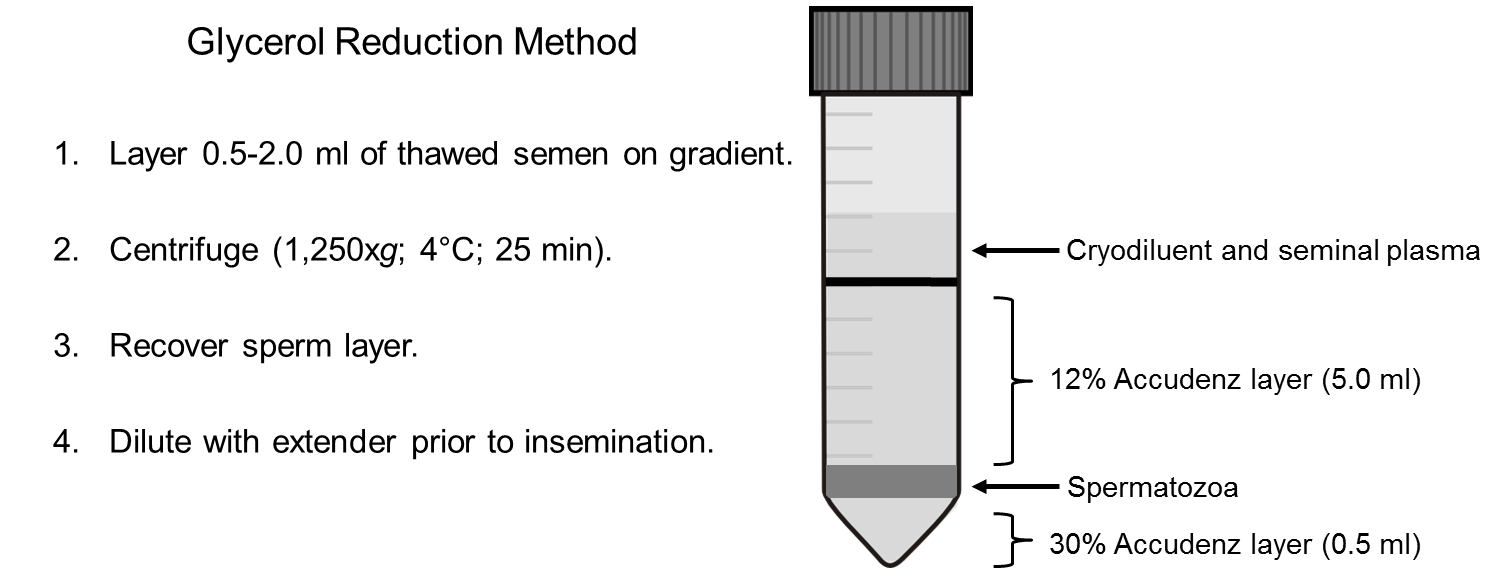
Accudenz centrifugation
Prepare discontinuous gradients in 15-mL conical centrifuge tubes by depositing 0.5 mL of 30% (wt/vol) Accudenz (Accudenz, Accurate Chemical & Scientific Corporation, Westbury, NY) under a 5-mL volume of 12% (wt/vol) Accudenz, using the stock solutions (recipe below) described by McLean et al. (1998) and Long and Kulkarni (2004).
Layer glycerolized semen (≤ 1 mL) on the discontinuous gradient and centrifuge the samples for 25 min at 1,250 × g at 4°C. A centrifuge brake should not be used so that the isolated sperm are not disrupted within the column.
Following centrifugation, the sperm are present at the interface of the 12 and 30% layers. Remove the upper gradient layer first, followed by the 30% layer using a new pipette tip so that only the sperm layer remains in the centrifuge tube.
Dilute the sperm pellet with up to 1 mL of extender, depending on the sperm volume, prior to recovery of the sperm. See Figure 1, below.
Determine the sperm volume and concentration.
Collect samples as described previously and immediately dilute 1:1 (vol:vol) with 5 °C glycerol- free Lake’s diluent (Lake and Stewart, 1978; recipe below).
Place diluted samples in a rack on ice and transport (<15 min) to a 5 °C room.
Dilute samples 1:2 (vol:vol) with Lake’s diluent containing DMA (6% final concentration; Blesbois et al., 2007) and loaded into 0.5-mL straws.
Cryopreserve samples using the box freezing method: Samples are placed on a rack and frozen in liquid nitrogen vapor (1.0 cm above liquid nitrogen) for 7 min (cooling rate: 59 °C/min; Blesbois et al., 2007).
Plunge samples into the liquid nitrogen for storage.
Thaw samples frozen using DMA in a 50 °C water bath for 20 s (Blesbois et al., 2007).
Inseminations using 200 x 106 sperm are used for either vaginal or intramagnal inseminations. Perform intramagnal insemination according to Song and Silversides (2007).
Anaesthetize hens using an injection of 0.15 mL to 0.20 mL (depending on the size of the bird) of xylazine (20 mg/mL) into the brachial vein.
Place the hen on her right side, draw up her left leg and remove the feathers overlying the left abdominal wall.
Wash the area around the site of the surgery with 70% ethanol.
Make an incision (approx. 2.5 cm) in the skin between the thigh and the breast close to the last rib.
Make a second incision (approx. 1 cm) in the thinnest section of the underlying muscle.
Using a retractor, expose the magnum which lies alongside the body wall in this area of the abdomen.
Hold a section of the magnum with large forceps and inject the sperm suspension using a 1 mL syringe equipped with a 20-gauge needle.
Return the exposed loop of the magnum to the peritoneal cavity and close the skin using continuous sutures.
Low Lakes Temperature (LLT) Medium without penetrating cryoprotectants 169.1mM Sodium glutamate-monosodium salt
33.3mM Fructose
4mM Magnesium Acetate (4H20) 62mM Sodium Acetate (Anhydrous) 3.7mM Potassium citrate
pH 7.5
LLT with Glycerol
84% LLT medium (by volume) 16% glycerol (by volume)
--OR--
LLT with DMA
Identical to the LLT medium except it includes 9% DMA (by volume)
Accudenz Stock Solutions (from McLean et al., 1998) for glycerol removal
Four solutions must be prepared to create the Accudenz columns:
30% Accudenz solution: 30% wt/vol Accudenz dissolved in 3 mM KCl + 5 mM TES (pH 7.4) TES buffer: 50 mM TES + 130 mM NaCl (pH 7.4; 315 mOsm)
Diluted TES buffer: TES buffer diluted to 275 mOsm with distilled, deionized water
12% Accudenz solution: dilute the 30% Accudenz solution 2.5x with the Diluted TES buffer. Figure 1. Graphic representation of Accudenz discontinuous centrifugation gradient
Blesbois, E., F. Seigneurin, I. Grasseau, C. Limouzin, J. Besnard, D. Gourichon, G. Coquerelle,
P. Rault, and M. Tixier-Boichard. 2007. Semen cryopreservation for ex situ management of genetic diversity in chicken: Creation of the French avian cryobank. Poult. Sci. 86:555–564.
Burrows, W. H., and J. P. Quinn. 1937. Collection of spermatozoa from the domestic fowl and turkey. Poult. Sci. 16:19–24.
Lake, P. E., and J. M. Stewart. 1978. Preservation of fowl semen in liquid nitrogen–An improved method. Br. Poult. Sci. 19:187–194.
Long J.A., and G. Kulkarni. 2004. An effective method for improving the fertility of glycerol- exposed poultry semen. Poult Sci. 83:1594-601.
McLean, D. J., A. J. Feltmann, and D. P. Froman. 1998. Transfer of sperm into a chemically defined environment by centrifugation through 12% (wt/vol) Accudenz. Poult. Sci. 77:163–168.
Phillips, J. J., R. K. Bramwell, and J. K. Graham. 1996. Cryopreservation of rooster sperm using methyl cellulose. Poult. Sci. 75:915–923.
Purdy, P.H., Y. Song, F.G. Silversides and H.D. Blackburn. 2009. Evaluation of glycerol removal techniques, cryoprotectants, and insemination methods for cryopreserving rooster sperm with implications of regeneration of breed or line or both. Poult. Sci. 88:2184-2191.
Song, Y., and F. G. Silversides. 2007. Heterotopic transplantation of testes in newly hatched chicken and production of viable offspring via intramagnal insemination. Biol. Reprod. 76:598– 603.
Tajima, A., E. F. Graham, and D. M. Hawkins. 1989. Estimation of the relative fertilizing ability of frozen chicken spermatozoa using a heterospermic competition method. J. Reprod. Fertil.
85:1–5.
Versions: February 2017, April 2020